Chemists just broke a 100-year-old rule and say it's time to rewrite the textbooks
31 ต.ค. 67
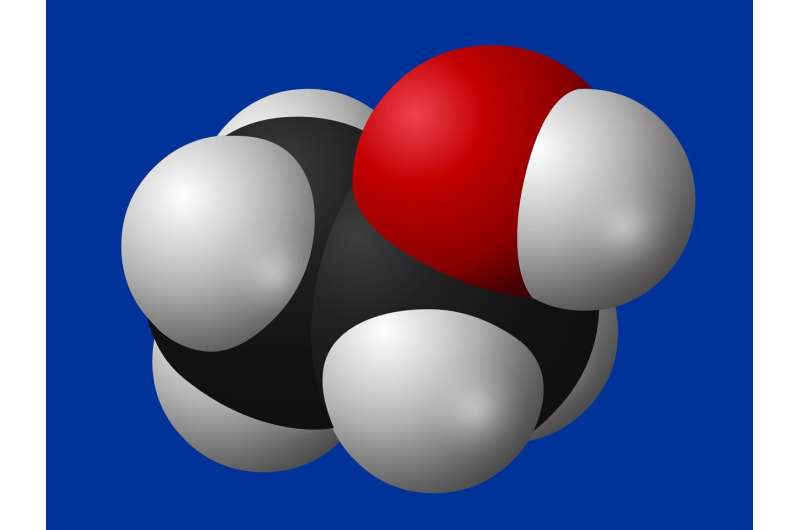
UCLA chemists have found a big problem with a fundamental rule of organic chemistry that has been around for 100 years—it's just not true. And they say, It's time to rewrite the textbooks. Organic molecules, those made primarily of carbon, are characterized by having specific shapes and arrangements of atoms. Molecules known as olefins have double bonds, or alkenes, between two carbon atoms. The atoms, and those attached to them, ordinarily lie in the same 3D plane. Molecules that deviate from this geometry are uncommon. The rule in question, known as Bredt's rule in textbooks, was reported in 1924. It states that molecules cannot have a carbon-carbon double bond at the ring junction of a bridged bicyclic molecule, also known as the "bridgehead" position. The double bond on these structures would have distorted, twisted geometrical shapes that deviate from the rigid geometry of alkenes taught in textbooks. .....
แหล่งที่มา
Phys.org
แท็ก